Kathleen Munson -
I returned from Hawai’i in mid-December with 700 bottles of seawater.
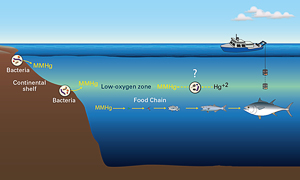
The bottles hold what I hope are solutions to an abiding mystery. In the middle of the ocean, waters at depths ranging from 100 to 1,000 meters contain high concentrations of the toxic form of mercury that accumulates in fish. Nobody knows why it’s there or where it comes from.
Mercury is unique among metals because it easily changes form between gas, liquid, and solid. It also exists in various chemical forms when it is dissolved in water.
One of those forms is monomethylmercury, which is found at high concentrations in the tissue of some fish and can have detrimental health impacts on people.
In particular, monomethylmercury hinders the neurological development of fetuses in pregnant women who eat large amounts of these fish.
However, monomethylmercury is very different chemically from the elemental mercury that is released from the burning of coal. The pathway between smokestack and fish is full of mysteries.
One mystery is where and how elemental mercury (Hg) takes on a methyl group (CH3) and is transformed into monomethylmercury (HgCH3) in marine environments.
Scientists do not know whether the monomethylmercury in the open ocean is made on location or whether it is brought into the ocean from the shore by currents.
The answer may lie within my bottles. Only a few scientists have measured monomethylmercury in the ocean, and they have found that the highest concentrations of monomethylmercury occur at mid-water ocean depths where oxygen concentrations are lowest.
These low-oxygen zones are created when plants and animals that grow at the ocean surface die.
They sink to mid-water depths and decompose, releasing large amounts of organic material to be recycled by marine bacteria. In that process, the bacteria use up oxygen.
This creates a complex environment where many different factors could trigger mercury methylation.
Posted via http://maritime-news.posterous.com Maritime-News posterous
.jpg)
No comments:
Post a Comment